In brief
Beginning December 29, 2023, the Modernization of Cosmetic Regulation Act of 2022 (MoCRA), which was signed into law last year to establish a more strengthened US Food and Drug Administration (FDA) regulatory framework to ensure the safety of cosmetic products,1 will begin to take effect. Like with cosmetic application, prepping for full coverage to comply with the new law is important.
In this first part of our upcoming series of articles on cosmetics, we will cover how to prepare for compliance with the new law, including an overview of the new FDA regulatory requirements and their implementation deadlines in the chart below.

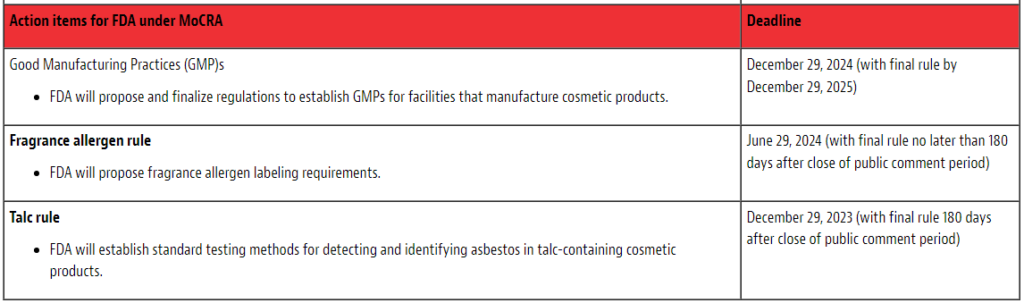
It is important to note that MoCRA will exempt certain small business from some of the regulatory requirements including GMP, registration, and product listing. However, as discussed in more detail below, such exemptions would not apply if the cosmetic products may potentially present higher risk to consumers. Exemptions also exist for certain products and facilities that are subject to requirements for drugs and devices.
In more detail
Facility registration and product listing requirements
Step 1: Prep your business
Prior to full coverage application, the first step is to prep to determine if the cosmetic registration and product listing requirements apply to the particular business.
- The registration requirements apply to cosmetics facilities that manufacture or process cosmetic products distributed in the United States. The term “facility” generally does not include the following:
- Beauty shops and salons
- Cosmetic product retailers, including individual sales representatives, direct sellers, retail distribution facilities, and pharmacies
- Entities (such as hotels and airlines) that provide complimentary cosmetic products to customers incidental to other services
- Trade shows and other venues where cosmetic product samples are provided free of charge
- An establishment that manufactures or processes cosmetic products that are solely for use in research or evaluation
- Facilities that solely perform labeling, relabeling, packaging, repackaging, holding and/or distribution are also not required to register
- There are also certain exemptions that apply to small businesses whose average gross annual sales in the US of cosmetic products for the past three years is less than USD 1,000,000, adjusted for inflation, and who do not engage in the manufacturing or processing of the following:
- Cosmetic products that regularly come into contact with mucus membrane of the eye under conditions of use that are customary or usual
- Cosmetic products that are injected
- Cosmetic products that are intended for internal use
- Cosmetic products that are intended to alter appear acne for more than 24 hours under conditions of use that are customary or usual and removal by the consumer is not part of such conditions of use that are customary or usual
Step 2: Prime your establishment with the proper foundation
For existing facilities that manufactured or processed cosmetic products for distribution in the United States at the time of enactment of MoCRA (December 29, 2022), registration is required to be submitted no later than December 29, 2023. For new facilities that began to manufacture or process cosmetic products for distribution in the United States after December 29, 2022, registration is required within 60 days of first engaging in a regulated activity or 60 days after December 29, 2023, whichever is later. The information required for registration includes:
- Facility’s name, physical address, email address, and telephone number
- US agent of the facility contact and electronic contact information, if a foreign facility
- Facility registration number, if previously assigned
- All brand names under which cosmetic products manufactured or processed in the facility are sold
- The product category or categories and responsible person for each
A “responsible person,” meaning the manufacturer, packer, or distributor of a cosmetic product whose name appears on the label,2 is also required to submit a product listing to list each cosmetic product, including the product’s ingredients. For cosmetic products marketed for distribution in the United States at the time of enactment of MoCRA (December 29, 2022), the product listing must be submitted no later than December 29, 2023. For cosmetic products marketed for distribution in the United States after December 29, 2022, product listing must be submitted within 120 days of marketing such product in interstate commerce. The information required for product listing is as follows:
- The facility registration number of each facility where the cosmetic product is manufactured or processed
- The name and contact number of the responsible person and the name for the cosmetic product, as such name appears on the label
- The applicable cosmetic category or categories for the cosmetic product
- A list of ingredients in the cosmetic product, including any fragrances, flavors, or colors, with each ingredient identified by the name
- The product listing number, if previously assigned
Step 3: Tips for lasting coverage
Establishments required to register a facility under MoCRA are required to renew the registration biennially. FDA should be notified of changes to information in the registration within 60 days of the change. Updates to cosmetic product listing shall be made annually (to give it a fresh look).
- FDA is currently developing a new legal framework for cosmetic regulation, which, among other things, would include the submission of the facility registrations and product listings and we expect further updates regarding its forthcoming availability to be published soon.
- The new law will begin to take effect December 29, 2023. To avoid any disruptions to trade, especially for cosmetic products that will be imported into the United States, companies should proactively establish compliance with the new regulatory requirements and closely monitor the agency’s implementation of MoCRA.
- MoCRA directs the FDA to establish cGMP regulations for cosmetic products. FDA will issue a proposed rule on GMPs by December 29, 2024 (with a final rule by December 29, 2025). Cosmetic products manufactured or processed under conditions that do not meet the GMPs will be deemed adulterated. FDA will host a listening session on June 1, 2023, to consult cosmetics manufacturers and other experts to inform its efforts in developing these regulations. Information regarding the listening session is available here. The next article in this series will provide an overview of the topics covered during the FDA GMPs listening session.
* * * * *
For further information and to discuss what this development might mean for you, please get in touch with the Baker McKenzie contacts provided above.
1 H.R. 2617-1389, Subtitle E – “Modernization of Cosmetics Regulation Act of 2022” (MoCRA), www.congress.gov/117/bills/hr2617/BILLS-117hr2617enr.pdf.
2 H.R. 2617-1389, Subtitle E – “Modernization of Cosmetics Regulation Act of 2022” (MoCRA), sec. 604.






